Immediate replacement of failed dental implants owing to periimplantitis
Link: https://www.dtscience.com/immediate-replacement-of-failed-dental-implants-owing-to-periimplantitis
September 15, 2015 / Categories: Digital Dentistry, Implant Dentistry
Anitua, Eduardo
Hamdan Alkhraisat, Mohammad
Tejero, Ricardo
Introduction
The high predictability of dental implants makes them the first choice for replacing missing teeth.132 This, in addition to the long-term success of implant-supported fixed prostheses,4 results in the wide acceptance of implant therapy among the general population.
New improvements in clinical protocols can increase the predictability of implant therapy further and reduce rehabilitation time and cost. One such improvement is the graftless rehabilitation of missing teeth. Lazzara et al. have introduced the concept of immediate implant placement after tooth extraction.5 This procedure results in a reduction in the number of surgical procedures and in the time required to complete oral rehabilitation. 67 Also, immediate implant placement is one of the surgical procedures by which to achieve alveolar ridge preservation.8
Published data document the high success rate of immediate implant placement and support the predictability of the technique in the absence of periapical lesions.910111213 Even in the presence of periapical infection, recent research has shown that immediate placement of dental implants is possible provided there is adequate socket cleaning and decontamination.141516 In a recent randomized clinical trial, Montoya-Salazar et al. studied the influence of periapical infection on the success rate of immediately placed dental implants after tooth extraction.17 The infected sockets were curetted and decontaminated before implant placement.10 In the group of infected sockets, all implants placed were successfully osseointegrated and loaded. The three-year survival rate was 94.44% with no significant differences when compared with the noninfected socket group.18
Periimplant mucositis and periimplantitis are inflammatory diseases of bacterial origin, but bone loss only occurs in the case of periimplantitis.19 The prevalence of periimplantitis varies between different studies and a prevalence (implant based) of 6.6–36.6% has been reported.2021222324 Dental implant extraction may be indicated in cases of advanced bone loss around the implant. In these cases, could immediate implant replacement be considered?
No study has reported on immediate implant placement after the extraction of infected dental implants. This dearth could be related to the need for a predictable technique that permits conservative implant extraction that preserves most of the viable soft and hard tissue. At the same time, the technique should not damage the bony walls of the socket and thereby compromise the osseointegration of the new dental implant. A kit for implant extraction has been developed to fulfill the above-mentioned requirements and to enhance the possibility of achieving adequate implant stability.2526
A clinical protocol that aims to decrease the bacterial load by curetting and decontamination of the socket, maintain the regenerative capacity of the surrounding alveolar walls, and achieve primary stability would result in favorable outcomes for immediate replacement of failed dental implants. In this article, we analyze the outcomes of this clinical protocol. To that end, failed, nonmobile, infected dental implants were extracted using an implant extraction kit and new implants were immediately placed in replacement of these. Plasma rich in growth factors was placed in the explantation socket before implant placement. The extracted dental implants were analyzed under a scanning electron microscope and the patients were followed for four years.
Materials & methods
Outcome criteria
In order to achieve the objectives of the study, demographic and anamnesis data were obtained from the patients’ records. Implant failure was defined as any implant lost owing to failure to achieve osseointegration or to loss of acquired osseointegration. The patient was the statistical unit for the description of demographic data. The implant was the statistical unit for the statistical description of implant location and removal torque. For the new implants, data on insertion torque, failure and marginal bone loss were collected. Implant length was used as a reference to calibrate the linear measurements on the digital panoramic radiograph. Implant survival rate was analyzed using the Kaplan–Meier method. All the statistical analyses were performed using the SPSS for Windows statistical software package (Version 15.0; SPSS, Chicago, Ill., U.S.).
Surgical protocol
Fig. 2
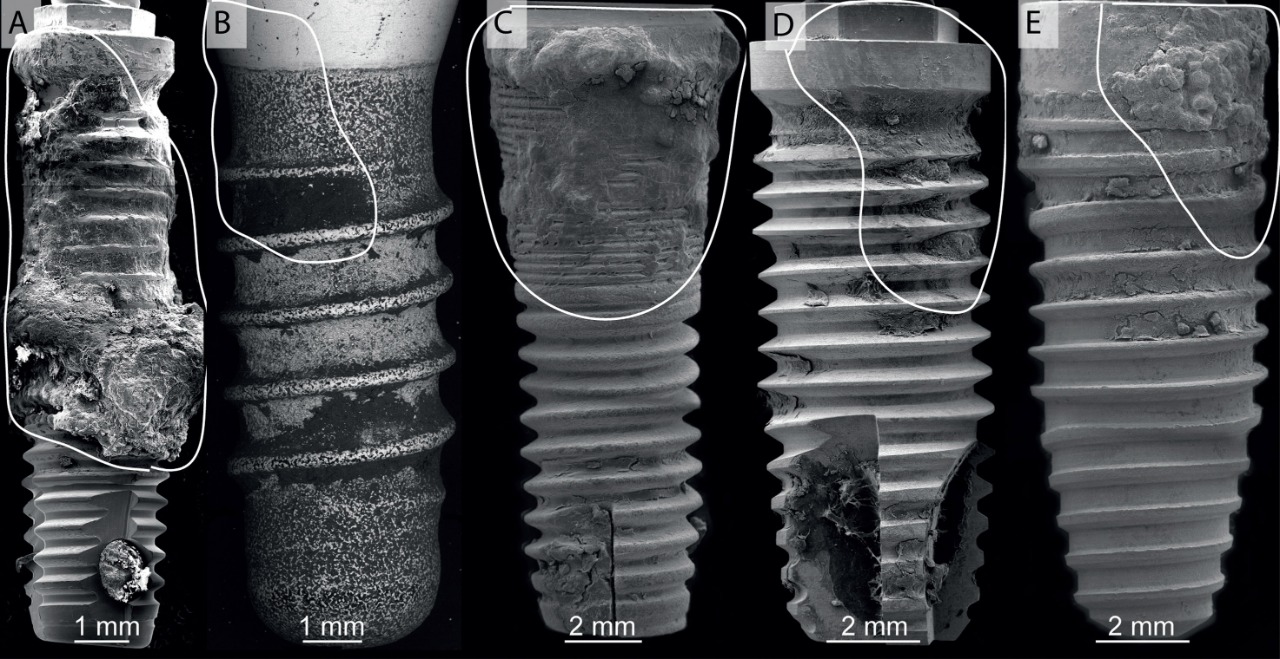
Scanning electron micrographs of five implants removed owing to periimplantitis. All of the implants showed clear signs of bacterial contamination and plaque formation, accompanied by vertical bone loss. Full implant images have been reconstructed using three to five scanning electron micrographs. The areas where bone resorption around the implants occurred are highlighted (white line). A scale bar is indicated for every implant image.
In all of the patients, the same surgical protocol was followed. All of the patients received prophylactic antibiotic medication before and after surgery. Infiltrative anesthesia was administered and incisions were made to elevate a full-thickness flap (Fig. 1). Implant explantation was carried out using an implant extraction kit (BTI Biotechnology Institute, Vitoria, Spain). A ratchet was first engaged into the implant connection and the removal torque was exerted by a wrench in a counterclockwise direction, maintaining a perpendicular position of the assembly in relation to the implant platform (Fig. 1).2728
After implant removal, the explantation socket was carefully curetted to remove any granulation tissue and immediate placement of a new implant was performed only in those sockets in which the four bony walls were preserved (Fig. 1). Bone drilling for placement of the new implant was performed to remove only 0.2– 0.5mm of bone. An implant with a wider diameter than that of the failed implant was then placed using a surgical motor set at an insertion torque of 25N cm and the implant placement was then continued manually to finish (Fig. 1). Activated fraction 2 of plasma rich in growth factors (PRGF-Endoret; BTI Biotechnology Institute, Vitoria, Spain) was placed in the socket before implant placement. The surgical site was then covered with a fibrin membrane before flap closure.
In order to obtain plasma rich in growth factors,29 peripheral blood was extracted by venipuncture into two 9ml extraction tubes containing 3.8% sodium citrate (BTI Biotechnology Institute, Vitoria, Spain) and processed according to the manufacturer’s instructions. To activate platelets and fibrin formation, 50μl of calcium chloride solution (PRGF Activator, BTI Biotechnology Institute, Vitoria, Spain) per milliliter of plasma was employed. Activated fraction 1 (platelet count comparable to the peripheral blood) was employed to prepare a fibrin membrane that was compressed (Fibrin compactor, BTI Biotechnology Institute, Vitoria, Spain) to provide a thin and consistent membrane to cover the surgical site before flap repositioning and suturing with a 5-0 monofilament nylon suture. Activated fraction 2 (platelet count two to three times higher than peripheral blood) was injected into the implant bed and was used to humidify the dental implants before placement. Followup visits were scheduled to remove sutures,detect any surgical complications and fabricate the implant-supported prosthesis.
Scanning electron microscopy
The extracted implants were studied under a scanning electron microscope (SEM, Quanta 200FEG, FEI, Eindhoven, Netherlands). Owing to the presence of organic components on the surface after explantation, the samples were fixed with a 2.5% glutaraldehyde solution (Sigma-Aldrich, St. Louis, Mo., U.S.) in phosphatebuffered saline (PBS, Sigma-Aldrich, St. Louis, Mo., U.S.) for 8h. The samples were then dehydrated by sequential immersion in serial diluted solutions of 0, 10, 30, 50, 70, 90 and 100% v/v of ethanol in water. Dehydrated samples were then air-dried, carbon-coated in a sample preparation chamber with a sputtering system (Gatan Alto 1000E, Gatan, Abingdon, UK) and examined by SEM. Images were taken at 20 kV acceleration voltage. The SEM-attached energy- dispersive X-ray unit served to analyze the elemental composition of the surface remnants.
Results
Seven patients with nine dental implants failed owing to periimplantitis were treated according to the previously described protocol. Six patients were females and the mean age was 61 ± 4 years. All patients were nonsmokers.
Six of the failed dental implants were in the maxillae. Four of the maxillary implants were in the anterior region and all of the mandibular implants were in the posterior region. The average extraction torque of the failed dental implants was 162 ± 41 N cm.
Fig. 3
Scanning electron micrographs showing details of the surfaces of the removed implants shown in Figure 2. In some cases, EDX was performed to determine the composition of particles found on the titanium (Ti) surfaces. Several explants showed abundant microbial colonization and biofilm formation (b–h). In some cases, attempts at decontamination could be traced back to the surface of the implants: In a, we performed EDX spot analysis of the particles found on the surface and found that they corresponded to surgical tools (stainless steel: Fe, Cr). Bacterial accumulation was preferential in the rough parts of the implant surfaces (arrows in b and g).
All of the explanted implants were analyzed by scanning electron microscopy. Figures 2 and 3 show representative sets of SEM images of the explanted implants. All of the implants were scanned completely at several magnifications. The lower magnification was used to obtain a general image of each of the implants extracted (Fig. 2). In these general images, traces of dental plaque can clearly be observed at the coronal parts of the implants. The area depicted over the implants (white line) corresponds to vertical bone defects detected before implant extraction. By increasing the magnification, details such as bacterial arrangements could be detected (Fig. 3). These were mainly cocci (Fig. 3: g & h) and bacilli (Fig. 3: b–f), although more sensitive techniques are needed to correctly identify the particular bacterial taxonomy. Biofilms disrupted by dehydration during the preparation of the samples could also be clearly identified (Fig. 3: d). In a in Figure 3, energy-dispersive X-ray spectroscopy (EDX) showed the presence of residue of inorganic materials on the implant surface, mainly iron and chrome. These particles could come from stainless-steel surgical tools used to attempt to eliminate the plaque adhering to the surface. From the image, we can clearly see that not only did the biofilm remain on the surface, but these procedures also left contaminants on the implant surface. In b in Figure 3, it can be seen how the plaque preferentially formed on the rougher parts of the surface. Overall, the evaluation of both the post-extraction sockets and the SEM images of the implant surfaces found that most of the dental plaque remained adhered to the removed implant surfaces.
New dental implants were immediately placed at a torque of 36 ± 16 N cm. Only two implants were placed at a torque of < 25 N cm. Two short implants of 5.5 mm × 5.5mm and 5.5 mm × 7.5 mm were placed. Three implants were 8.5 mm in length and had a diameter of 4.0, 4.5 and 5.5 mm, respectively. The rest of the implants were 10.0–13.0 mm in length and 3.75–5.0 mm in diameter, respectively. The nine implants gave support to a single crown, four partial fixed prostheses and four complete fixed prostheses. All of the partial and complete fixed prostheses were screw retained. The implant loading was immediate for the single crown and delayed for the partial and complete fixed prostheses. The implants were followed for 50 ± 2 months after placement (range: 48–52 months) and 43 ± 3 months after loading (range: 40–48 months). No implant failure was registered during this period. The mesial bone loss was 1.0 ± 0.8 mm and the distal bone loss was 1.0 ± 0.8 mm. The marginal bone loss was measured on radiographs taken after 40 ± 6 months of implant loading. Figure 4 shows a case that was treated according to the described protocol and followed for four years after implant placement.
Discussion
The results of this study support the immediate replacement of failed dental implants after extraction. The clinical protocol followed for the management of failed dental implants would enhance the possibility of osseointegration of dental implants placed in infected sites.
The positive outcomes of this protocol could be related to the decrease in the bacterial load through the removal of the infected implant. The SEM analyses showed that bacterial plaque still adhered to the implant surface upon removal, and this represents a first step in the cleaning of the extraction socket. Adequate socket curettage to remove any granulation tissue and the drilling of the socket would additionally contribute to the mechanical decontamination of the socket. Furthermore, placement of PRGF-Endoret in the socket could have had an antimicrobial effect. It has been reported that PRGF-Endoret has antimicrobial effects against Candida albicans, Enterococcus faecalis, Streptococcus agalactiae, Streptococcus oralis, Staphylococcus aureus and Staphylococcus epidermidis.3031 All of these measures would reduce the risk of infection and early implant failure.
Implant primary stability is crucial for implant osseointegration and is the result of mechanical anchoring (direct contact) of the implant to the host bone.32 Implant primary stability serves to prevent excessive implant micromovements and thus permit implant osseointegration.33 The insertion torque of the dental implants placed in this study was 36 ± 16 N cm. Engelke et al. have concluded that an insertion torque of > 30 N cm is advisable to obtain adequate primary stability and a torque of ≤ 11 N cm is considered a risk factor that increases the likelihood of implant failure.34
Different methods to remove osseointegrated dental implants have been described. Some of them include trephining a bone block in which the dental implant is present and the use of a thin bur at low speed with irrigation to separate the implant from the surrounding bone.3536 These methods have the limitation of being traumatic and of jeopardizing the explantation socket for future implant placement. In this study, the use of an implant extraction kit was efficient and minimally invasive in removing dental implants while preserving the alveolar bone. This made it feasible to replace the failed implant immediately. This immediate replacement of failed implants reduced the number of surgical procedures required to treat the patient.
In a recent study, 81 patients were treated with the same implant extraction kit to remove 158 nonmobile implants from the maxillae and the mandible.37 With the kit, the conservation of hard and soft tissue is possible and implant failure can be resolved within a shorter period and at reduced cost by avoiding advanced tissue regeneration techniques.
Conclusion
Atraumatic implant explantation permitted the preservation of viable tissue and the immediate placement of a new implant. The implant survival and marginal bone loss outcomes would support the immediate placement of dental implants in a socket affected by periimplantitis.