HUMAN HEALTH RISK ASSESSMENT FOR ALUMINIUM, ALUMINIUM OXIDE, AND ALUMINIUM HYDROXIDE
Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2782734/
J Toxicol Environ Health B Crit Rev. Author manuscript; available in PMC 2009 Nov 25.
Published in final edited form as: J Toxicol Environ Health B Crit Rev. 2007; 10(Suppl 1): 1–269. doi: 10.1080/10937400701597766 PMCID: PMC2782734 NIHMSID: NIHMS33559 PMID: 18085482
Daniel Krewski,1,2 Robert A Yokel,3 Evert Nieboer,4 David Borchelt,5 Joshua Cohen,6 Jean Harry,7 Sam Kacew,2,8 Joan Lindsay,9 Amal M Mahfouz,10 and Virginie Rondeau11
EXECUTIVE SUMMARY
Identity, Physical and Chemical Properties, Analytical Methods
A compendium is provided of aluminium compounds used in industrial settings, and as pharmaceuticals, food additives, cosmetics and as other household products. Most aluminium compounds are solids exhibiting high melting points. The solubility of aluminium salts is governed by pH, because the aluminium(III)-cation (Al3+) has a strong affinity for the hydroxide ion, which promotes precipitation. Like Mg2+ and Ca2+ ions, Al3+ in most situations seeks out complexing agents with oxygen-atom donor sites such as carboxylate and phosphate groups, including in biological systems. Aluminium oxides, hydroxides and oxyhydroxides occur in numerous crystallographic forms, which exhibit different surface properties. Few compounds of aluminium are classified in Annex 1 of the European Economic Union Council (EEC) Directive 67/1548, with aluminium powder and sodium aluminium fluoride (cryolite) as examples of exceptions, as well as compounds in which the anion renders them reactive such as aluminium phosphide. And finally, the more recent analytical methods available for the study of chemical speciation in solids and solution, and for quantitative analysis, have been applied to the determination of aluminium and the identification of its various forms.
Sources of Human Exposure
Aluminium and its compounds comprise about 8% of the Earth’s surface; aluminium occurs naturally in silicates, cryolite, and bauxite rock. Natural processes account for most of the redistribution of aluminium in the environment. Acidic precipitation mobilizes aluminium from natural sources, and direct anthropogenic releases of aluminium compounds associated with industrial processes occur mainly to air. Certain uses lead to the presence of aluminium in drinking water and foodstuffs.
Bauxite is the most important raw material used in the production of aluminium. Bauxite is refined to produce alumina from which aluminium metal is recovered by electrolytic reduction; aluminium is also recycled from scrap. Aluminium hydroxide is produced from bauxite. In 2004, primary aluminium was being produced in 41 countries, the largest producers being China, Russia, Canada and the United States. In that year, worldwide production of primary aluminium, alumina and aluminium hydroxide reached about 30, 63, and 5 million tonnes per annum, respectively. More than 7 million tonnes of aluminium is recovered annually from recycled old scrap.
The largest markets for aluminium metal and its alloys are in transportation, building and construction, packaging and in electrical equipment. Transportation uses are one of the fastest growing areas for aluminium use. Aluminium powders are used in pigments and paints, fuel additives, explosives and propellants. Aluminium oxides are used as food additives and in the manufacture of, for example, abrasives, refractories, ceramics, electrical insulators, catalysts, paper, spark plugs, light bulbs, artificial gems, alloys, glass and heat resistant fibres. Aluminium hydroxide is used widely in pharmaceutical and personal care products. Food related uses of aluminium compounds include preservatives, fillers, colouring agents, anti-caking agents, emulsifiers and baking powders; soy-based infant formula can contain aluminium. Natural aluminium minerals especially bentonite and zeolite are used in water purification, sugar refining, brewing and paper industries.
Aluminium has not been classified with respect to carcinogenicity; however, “aluminium production” has been classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC) (for further explanation, please see Effects on Humans, Effects from Occupational Exposure, Cancer). Occupational limits exist in several countries for exposures to aluminium dust and aluminium oxide. For non-occupational environments, limits have been set for intake in foods and drinking water; the latter are based on aesthetic or practical, rather than health, considerations.
Environmental Levels and Human Exposure
Aluminium may be designated as crustal in origin, and thus surface soils at uncontaminated sites constitute a source of soluble aluminium species in surface water and aluminium-containing particulates in sediments and ambient-air aerosols. Not surprisingly, the latter are present extensively in air samples in agricultural communities and when road dust is extensive. Environmental acidification is known to mobilize aluminium from land to aquatic environments. Interestingly, aluminium levels and its various forms (species) are often similar in source water and after its treatment with potassium alum as a flocculent during drinking water purification.
Workers in the aluminium production and user industries, as well as aluminium welders, experience considerable exposures to the metal and/or its compounds. In absence of occupational exposures and chronic use of aluminium-containing antacids and buffered aspirin, food is the major intake source of aluminium, followed by drinking water. When considering bioavailability, namely the fraction that is actually taken up into the blood stream, food is again the primary uptake source for individuals not occupationally exposed. However, chronic use of antacids, buffered aspirins and other medical preparations would likely constitute the major uptake source, even when exposed at work.
Kinetics and Metabolism
Humans
The use of 26Al as a tracer and accelerator mass spectrometry has enabled safe studies of aluminium toxicokinetics with real exposure-relevant doses in humans. Aluminium bioavailability from occupational inhalation exposure is ~ 2% whereas oral aluminium bioavailability from water has been reported to be 0.1 to 0.4%. Oral aluminium bioavailability is increased by citrate, acidic pH, and uraemia and may be decreased by silicon-containing compounds. Oral aluminium bioavailability is also inversely related to iron status.
Oral aluminium bioavailability is greater from water than from aluminium hydroxide or sucralfate. Oral aluminium bioavailability from aluminium hydroxide is ≤ 0.1%, and is less with higher doses. Increased oral aluminium absorption has been suggested in Alzheimer’s disease (AD) and Down’s subjects. Oral aluminium bioavailability from the diet has been estimated to be ~ 0.1 to 0.3%, based on daily aluminium intake and urinary elimination. Results of a few studies with a controlled diet and tea are consistent with this estimate.
Steady state serum to whole blood aluminium concentrations are ~ equal. Slightly > 90% of plasma aluminium is associated with transferrin (Tf), ~ 7 to 8% with citrate, and < 1% with phosphate and hydroxide. Normal plasma aluminium concentration is believed to be 1 to 2 μg/L. Normal tissue aluminium concentrations are greater in lung (due to entrapment of particles from the environment) than bone than soft tissues. Approximately 60, 25, 10, 3 and 1% of the aluminium body burden is in the bone, lung, muscle, liver and brain, respectively. Higher concentrations are seen in uraemia and higher still in dialysis encephalopathy.
Tissue aluminium concentration increases with age. Some studies have reported that the aluminium concentration in the bulk brain samples, neurofibrillary tangles (NFT) and plaques was higher in AD subjects than controls. Other studies have found no difference. Hair aluminium concentration has been described but its value as an indicator of aluminium body burden has not been demonstrated.
Greater than 95% of aluminium is eliminated by the kidney; ~ 2% in bile. Occupational aluminium exposure increases urinary more than plasma aluminium concentration above their normal levels. Depending on the type and route of exposure, aluminium clearance has been characterized as having multiple half-times and are estimated in hours, days, and years. Most of the Al was eliminated within the first week; the terminal half-life probably represents < 1% of the injected aluminium.
Biological monitoring of human aluminium exposure has been conducted with urine, which is thought to indicate recent exposure, and plasma, which is thought to better reflect the aluminium body burden and long-term exposure. However, neither is a very good predictor of the aluminium body burden, which is better estimated by bone aluminium, the desferrioxamine challenge test, or combined measurement of serum iPTH (parathyroid hormone) and the desferrioxamine test.
Serum aluminium > 30 μg/L in dialysis patients has been associated with osteomalacia and related disorders and > 80 μg/L associated with encephalopathy. Up to 5 mg/kg of desferrioxamine once or twice weekly has been shown to be safe and effective for long-term treatment of aluminium overload.
Animals
In studies of animals, pulmonary deposition of fly ash was 2 to 12% and was inversely related to particle size. Oral aluminium bioavailability from water appears to be ~ 0.3%. The very limited data available suggest oral aluminium bioavailability from food is less than from water.
Oral aluminium bioavailability is increased by citrate, and to a lesser extent, other carboxylic acids, increased solubility of the aluminium species, acidic pH, uraemia, increased dose of soluble aluminium species, and perhaps fluoride. Oral aluminium bioavailability is decreased by silicon-containing compounds. Oral aluminium bioavailability is also inversely related to iron, calcium and sodium status.
Absorption of aluminium from the gastrointestinal tract (GI) appears to be primarily in the distal intestine. There is evidence supporting several mechanisms of intestinal aluminium absorption, including sodium transport processes, an interaction with calcium uptake, and paracellular diffusion. Aluminium penetration of the skin is very shallow. Aluminium may be able to enter the brain from the nasal cavity by a direct route, bypassing systemic circulation, but convincing evidence is lacking. Absorption of aluminium from intramuscularly (i.m.) injected aluminium hydroxide and aluminiun phosphate adjuvants is significant, and may eventually be complete. Tissue aluminium concentration increases with age.
The volume of distribution (Vd) of aluminium is initially consistent with the blood volume, and then increases with time. Steady state serum to whole blood aluminium concentrations are ~ equal. Greater than 90% of serum aluminium is bound to Tf. Although aluminium has been reported in many intracellular compartments, concentrations were often greater in the nucleus. Ferritin can incorporate aluminium.
Following i.v. injection, ~ 0.001 to 0.01% of the aluminium dose enters each gram of brain and ~ 100-fold more each gram of bone. Brain aluminium uptake across the blood-brain barrier (BBB) may be mediated by Tf-receptor mediated endocytosis (TfR-ME) and a Tf-independent mechanism that may transport aluminium citrate. There appears to be a transporter that effluxes aluminium from the brain into blood. Aluminium distributes into the placenta, foetus, milk, hair, and can be quantified in all tissues and fluids. Greater than 95% of aluminium is eliminated by the kidney, probably by glomerular filtration. Less than 2% appears in bile.
Aluminium clearance is characterized by multiple half-lives (t½), suggesting multiple compartments. The terminal t½ from the lung is ~ 100 days and from the brain and other soft tissues > 100 days. Prolonged aluminium residence in the bone may account for the prolonged t½ observed in most organs, including the brain.
There are no published reports of physiologically based pharmacokinetic (PBPK) modelling of aluminium. A few models have been developed that incorporate the reported results of toxicokinetic studies with aluminium.
Effects on Laboratory Mammals and In Vitro Test Systems
Regardless of the duration of exposure, the toxicity attributed to aluminium is dependent upon the physiochemical properties (solubility, pH, bioavailability, etc.), type of aluminium preparation, route of administration, and physiological status (presence of renal dysfunction). Following oral exposure, aluminium distributes throughout the organism with accumulation in bone, kidneys and brain being of concern to humans with evidence of renal dysfunction, anemia or neurobehavioural alterations reported after excessive doses. The presence of aluminium in vaccines was found to be associated with macrophagic myofasciitis (MMF) at the site of i.m. injection. The toxicity of aluminium is affected by chelating agents and ligands although the mechanisms underlying toxicity remain unknown. However, it should be noted that only at excessive concentrations of aluminium are toxic manifestations seen and, hence aluminium is considered to possess a “low” potential for producing adverse effects.
Oral administration of aluminium did not affect reproductive capacity in males or females. Exposure to aluminium during gestation did not affect maternal health or development of the foetuses and neonates. Further, there was no evidence of teratogenic alterations in the foetuses of mothers fed dietary aluminium. Maternal dietary exposure to excessive amounts of aluminium during gestation and lactation resulted in neurobehavioural abnormalities in mouse offspring. At physiological concentrations the reproductive system does not appear to be a target for aluminium-induced effects; and if there is exposure during pregnancy, the growth and development of offspring of metal-treated mothers is not adversely affected.
The form of aluminium most often presented to tissues outside of the blood stream is expected to be bound to Tf. In brain, aluminium is prone to dissociate from Tf as a soluble citrate salt. Most cells of the central nervous system (CNS) express Tf receptor, and thus receptor-mediated uptake would be one mechanism by which aluminium could enter cells of the brain. Free flow endocytosis of aluminium citrate could be an alternative route of uptake. As outlined in Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, In Vivo Models, Neuropathology, there is at least one example of human pathology which is consistent with this mode of tissue exposure. Choroid plexus epithelia, cortical glia, and cortical neurons of patients exhibiting dialysis associated encephalopathy (DAE) develop intracellular argentophylllic granules that are lysosome-derived and intracytoplasmic. Uptake of aluminium-Tf complexes via receptor-mediated endocytosis would be expected to produce just such pathology. Whether aluminium, of any amount or speciation, escapes these compartments to impact on intracellular processes in humans is unknown. If relatively high doses produce pathology of such a distinctive nature, then it is reasonable to presume that lower doses of aluminium would follow similar pathways into the nervous system of humans.
In the studies of animals, it is important to note that a few reports have documented a pathologic accumulation of aluminium in intracellular lysosome-derived structures. Aluminium accumulation in lysosome-like cytoplasmic granules of retinal neurons in rats exposed to very high doses of aluminium was reported (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, In Vivo Models, Rodent Models of Aluminium Toxicity by Direct Injection). Severe atrophy of the retina and loss of photoreceptors was also noted. Similarly, another study noted intracellular accumulations of aluminium in the brain of rats feed diets high in aluminium. For CNS it seems likely that the mode of delivery to the tissue is through Tf-mediated uptake. From animal studies and the clear association of aluminium exposure and DAE, it is clear that high levels of aluminium in CNS can lead to neurotoxicity. From the current literature it remains difficult to assess what a concentration of aluminium in serum (chronic levels) correlates with neurotoxicity. The effects of aluminium on the developing nervous system have also not been thoroughly addressed.
In regards to mechanisms by which aluminium could play a role in AD, there are both direct and indirect modes of potential action. In a direct mode, aluminium could potentiate the aggregation of molecules known to form pathologic lesions in AD. There is evidence that aluminium can promote the aggregation of β-amyloid peptide in vitro. However, whether aluminium would dissociate from Tf at an appreciable rate and bind β-amyloid peptide in vivo is unclear. One study found no association between AD-like pathology and long-term ingestion of aluminium. Indeed in this study of older patients, the incidence of AD-associated pathology in patients with DAE was no different from controls. Although these studies would suggest that there is little direct evidence for an association between AD and aluminium, a study of transgenic mice that produce Alzheimer-type amyloid pathology noted that mice feed diets high in aluminium showed increased levels of amyloid (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, Alzheimer’s Disease). Moreover, it is well established in the rabbit that exposure to aluminium induces the formation of filamentous structures containing cytoplasmic neurofilament protein (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, Motor Neuron Disease). Therefore, it is difficult to determine how a life-time of exposure to aluminium might influence the development of Alzheimer-type pathology by affecting the folding or clearance of “at-risk” proteins such as β-amyloid, tau, and α-synuclein.
Apart from the potential that aluminium might interact directly with molecules implicated in AD and related neurodegenerative disorders, studies in animals have revealed potential mechanisms by which aluminium might indirectly impact on the function of the nervous system. In Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, Alzheimer’s Disease, studies are described that reported aluminium may affect levels of cholesterol, which has been suggested in numerous studies as a potential modulator or Alzheimer-type amyloid formation. Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, In Vivo Models, Rodent Models of Aluminium Toxicity by Direct Injection describes several studies that have reported elevated levels of markers of oxidative stress in animals exposed to aluminium. These studies suggest potential mechanisms by which long-term exposure to aluminium could be deleterious and could synergistically worsen cognitive abilities in individuals that have pathologic abnormalities associated with AD.
However, there has not been strong evidence from animal studies that aluminium directly modulates cognitive function. As described in Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity,Behavioural Studies of Laboratory Animals Exposed to Aluminium, there have been several studies that have examined the cognitive abilities of mice and rats exposed to aluminium. For the most part, these studies did not report profound cognitive impairment even when exposed to very high levels of aluminium. Therefore, it seems unlikely that aluminium might lower the threshold for AD by blunting cognitive ability of adults.
Outside of the nervous system, the data regarding the potential for alumimium to cause abnormalities is mixed. There is clear evidence that sustained exposure to high levels of aluminium can cause bone abnormalities. Aluminium is clearly deposited in bone at sites of new growth. Bones in animals exposed to aluminium may show increased weakness and increased brittleness. Deficiencies in calcium or magnesium may exacerbate the effects of aluminium. Aluminium overload leads to PTH suppression and with regards to the bone, may be associated with altered calcium homeostasis.
Aluminium may also have negative effects on hematopoiesis. However, these effects are relatively mild unless animals are deficient in iron. In this latter setting, there will be increased levels of free Tf, which can then bind aluminium and compete for Tf receptor; further limiting the amount of iron available for erythrogenesis. Aluminium may also interfere with the metabolism of other metals. On this latter point, the strongest data, meaning most reproducible, suggest that aluminium exposure can lead to increased excretion of phosphorous.
From the present data, however, it is difficult to determine what level of exposure poses a risk for human health or which systems are most vulnerable. Based on projections from studies in dogs, individuals with sustained aluminium levels in serum that are 10-fold higher than the average range, or 1-2 μg/L, may be at increased risk for bone abnormalities. The exposure levels at which other systems might be affected are more difficult to project, particularly when trying to assess risk for late-onset illnesses.
Although not reported in every study, the majority of studies that utilized high doses of aluminium reported significant reductions in weight gain, particularly in studies initiated in young animals. The physiologic basis for this outcome is unclear, but it was reported that animals exposed to high doses of aluminium in drinking water consumed less food. Whether general effects of aluminium on metabolic processes depress metabolism or reduce nutritional efficiency remains to be resolved.
Experimental aluminium inhalation has been shown to produce effects interpreted as alveolar proteinosis and lipid pneumonia. Inhalation of aluminium had some protective effect against quartz dust-induced fibrosis in some, but not all, studies. Intratracheal aluminium instillation produced nodular fibrosis. Aluminium is used as an adjuvant in vaccines and hyposensitization treatments to precipitate toxins and toxoids, enhance their antigenic properties and reduce their rate of absorption and elimination. Aluminium can produce aluminium-species-dependent dermal irritation.
Experimental animal studies have failed to demonstrate carcinogenicity attributed solely to aluminium compounds. Often the response reported is associated with a tissue response to a foreign body rather than a direct effect of aluminium exposure. This appeared to be consistent across various routes of exposure from inhalation to intraperitoneal (i.p.) injection.
In agreement with their non-carcinogenic activity, aluminium compounds failed to show positive results in most short-term mutagenic assays and animal experiments to determine genotoxic potential of aluminium compounds lead to contradictory results with suggestions of an anti-genotoxic potential.
There is little reported for aluminium compounds in the way of immunotoxicity. There may be an altered immune response to challenge following excess aluminium exposure and this may be influenced by the health and hormonal status of the dam with increased susceptibility to bacterial infection seen in pregnancy.
Effects on Humans
Occupational exposure
Occupational exposure to aluminium occurs during the refining of the primary metal and in secondary industries that use aluminium products. Several studies have reported adverse respiratory tract effects in aluminium industry employees. Asthma-like symptoms, known as potroom asthma, have been the most intensely investigated respiratory effect. Wheezing, dyspnea, and impaired lung function (typically assessed by measuring forced expiratory volume (FEV1) and forced volume capacity (FVC)) are the primary features of this disorder. Several cross-sectional, case-control and longitudinal studies have demonstrated increased frequency of adverse pulmonary effects in potroom workers as compared to non-exposed workers. The cause of potroom asthma has not been fully elucidated, but job specific exposure measurements based on personal sampling data and analysis of plasma levels suggests that exposure to fluorides may be an important determinant. There is some evidence to support that individuals with hay fever and individuals with elevated eosinophil counts are at increased risk of developing potroom asthma. Other studies did not find an association between allergic status and the development of symptoms. The respiratory problems documented in potroom aluminium workers are generally associated with toxic chemicals other than aluminium in the workplace. In contrast, exposure to aluminium powder is thought to be directly correlated with the development of pulmonary fibrosis in aluminium industry workers.
Adverse neurological outcomes as a result of occupational aluminium exposure have also been extensively investigated. Aluminium exposure in these studies was estimated in a number of different ways including; exposure grading for different job categories, determination of total body burden of aluminium, number of years working in the aluminium industry, and ever v.s. never worked in the aluminium industry. Occupational aluminium exposure was significantly correlated with a variety of neuropyschiatric symptoms including; loss of coordination, loss of memory, and problems with balance. Studies which specifically examined the relationship between AD and occupational aluminium exposure did not show any significant correlation. However, these studies are limited by methodological issues.
The occurrence of contact dermatitis and irritant dermatitis was reported in workers exposed to aluminium alloys and aluminium dust.
Several epidemiological studies have reported an increased risk of developing lung cancer or bladder cancer for workers in the aluminium industry, however, in all of these studies the risk has been attributed to the exposure to the PAHs generated during aluminium production rather than from exposure to aluminium compounds. Studies investigating the effects of occupational exposure to aluminium are limited by many methodological issues. Rarely is a worker exposed solely to aluminium containing compounds and exposure information is often not adequate to rule out other toxic substances as the cause of the observed effect. Small sample sizes, misclassification bias, selection of inappropriate comparison groups, and lack of information to control for confounding factors are common weaknesses in these occupational studies.
Changes typical of foreign body reaction, alveolar proteinosis and wall thickening, diffuse pulmonary fibrosis and interstitial emphysema, and some nodule formation but not to the extent of fibrosis caused by quartz dust were associated with occupational exposure in the aluminium industry. This was most severe in Germany during World War II, where industrial environments were heavily contaminated with airborne aluminium flake powder. Lower aluminium exposures contribute to Shaver’s disease, a pulmonary fibrosis seen in workers in bauxite refining or exposed to finely divided aluminium powders; and caused pneumoconiosis, fibrosis, and some cases of asthma.
Only one case-control study examined associations between genotype and the development of asthma for workers employed in a potroom. However this study with very low power did not find any association.
No reliable epidemiological studies exist to reach any conclusion on an association between occupational exposure to aluminium and fertility or developmental effects. No clear results have been obtained on gene-environment interactions.
Non-occupational exposure
The neurotoxic properties of aluminium are well established; however, the evidence surrounding the potential association between aluminium and neurological disorders in humans is much less clear. Aluminium exposure from drinking water has been extensively investigated in relation to the development of neurological disorders, including AD, due to the proposed enhanced bioavailability of aluminium in this form. The data surrounding this association is difficult to interpret due to the large variation in study designs and the highly variable quality of these studies. The majority, but not all, of epidemiological studies identified, reported a positive association between aluminium levels in drinking water and risk of cognitive impairment dementia, or AD. There is some evidence to suggest silica in drinking water is protective against the development of dementia. Fluoride has also been identified as having a potential protective effect. Many of the studies which have investigated the relationship between aluminium in drinking water supplies and the risk of developing AD are limited by methodological issues. These issues include: lack of individual exposure information, poor disease ascertainment, failure to adjust for important confounding factors, and small sample sizes. A recent study conducted in France is methodologically superior to the other studies conducted to date. The finding of a significant positive relationship between drinking water aluminium levels and the development of AD in this large prospective study, together with the finding of a positive relationship in a number of less methodologically sound studies, suggests that the association between aluminium and AD should be further investigated.
Regular consumers of antacids represent a unique subpopulation with heavy exposure to aluminium. A significantly elevated odds ratio for AD for regular antacid consumers compared to non-regular users was found; however, when only aluminium containing acids were analyzed there was no significant association. Other studies have not found a significant association between antacid use and AD. Little is known about the impact of aluminium-containing antacids in human pregnancy and lactation.
Evidence surrounding the relationship between aluminium in food and the risk of AD is very minimal. This may be a result of the difficulty in obtaining accurate exposure information in dietary studies. One small case control study found a positive relationship between the consumption of foods containing high levels of aluminium and the risk of developing AD. These results have not been confirmed in a larger investigation.
There is a large body of literature, mostly in the form of clinical reports, which documents the adverse effects of non-occupational aluminium exposure in individuals with impaired renal function. These patients are typically exposed to aluminium through dialysate fluid or medicinal sources. Anaemia, bone disease, and dialysis encephalopathy are the most commonly reported complications of aluminium exposure in this population.
Contact sensitivity to aluminium is very rare. Sensitization has occurred after injection of aluminium-adjuvant containing vaccines and pollen extracts, resulting in persistent granuloma at the injection site. These effects are much more frequent with aluminium hydroxide than aluminium phosphate adjuvants and more commonly seen following subcutaneous (s.c.) than i.m. injection. Less common is sensitivity during continuous application of aluminium-containing antiperspirants, topical aluminium application, and occupational exposure to aluminium dust and filings which result in recurrent eczema.
Only a few epidemiological studies with no clear results have been undertaken of the possible carcinogenic risks (such as breast cancer) of antiperspirants.
The exact genetic effects of Tf (a major transport protein for both iron and aluminium) itself or its interaction with aluminium remains unclear and has led to contradictory results.
As a result of inadvertent human poisoning with excessive amounts of aluminium, there are reports of damage to bone and CNS as target organs. Further, the administration of aluminium-containing vaccines for extended time periods was found to be associated with the development of MMF at the injection site. In the past, individuals with impaired renal function receiving dialysis were reported to be at greater risk for aluminium intoxication associated with contaminated replacement fluids. However, this incidence has diminished markedly in recent years with the use of non-contaminated fluid and replacement of high-dose antacid therapy with alternatives. Although infants and children may be at higher risk for toxicity due to aluminium, a causal relationship was not confirmed. Hence, it should be noted that only at excessive concentrations of aluminium are toxic manifestations seen in human sensitive subpopulations.
Conclusions
This report synthesizes data from relevant studies on potential health effects of exposure to aluminium to quantify risk using the four-step process specified by the National Research Council: 1) hazard identification, 2) exposure assessment, 3) dose-response assessment, and 4) risk characterization.
Hazard identification qualitatively identifies adverse effects by route of exposure, and determines whether those effects are likely in humans at some level of exposure, perhaps much greater than exposure levels experienced in the population of interest. It is important to note that the identification of effects that can be caused by aluminium says nothing about how likely those effects are at exposure levels in human populations. That probability depends on the level of exposure and the dose-response relationship. This report classified the weight of evidence for each exposure pathway and health effect as strong, modest, limited, or having no clear evidence (see Table 25). We concluded that there is strong evidence that aluminium can cause irritation following exposure via either inhalation or injection. Modest evidence of an effect exists for reproductive toxicity following oral exposure, for neurological toxicity following either oral or injection exposure, and for bone toxicity following injection exposure. All other effects were judged to be supported by either limited evidence or no clear evidence at all. Exposure assessment, dose-response assessment, and risk characterization were conducted for those effects for which the evidence was judged to be either strong or modest. The remainder of this section describes our findings for the general population, subpopulations at special risk, and occupationally-exposed populations.
General population
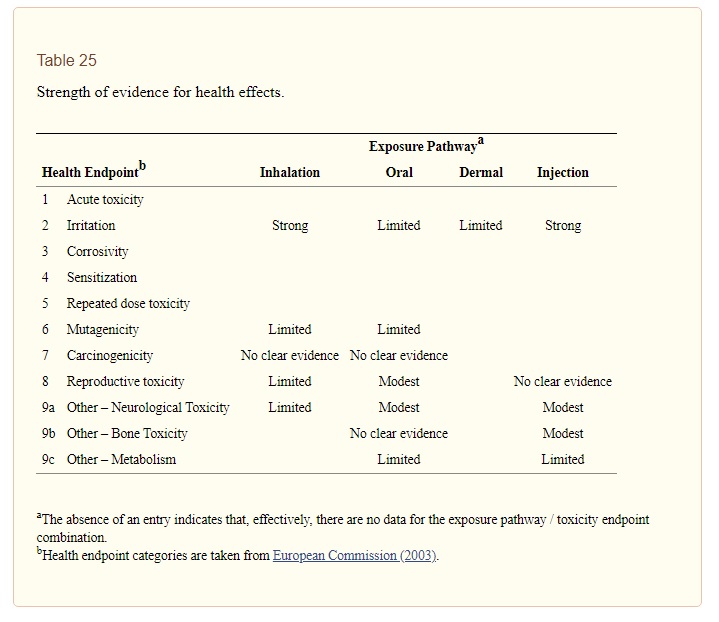
Exposure assessment quantified aluminium intake and uptake (i.e., absorption of aluminium into systemic circulation) for a variety of pathways (see Table 26). For the general population, intake of aluminium from food (7.2 mg/day for females and 8.6 mg/day for males) dominated that from drinking water (0.16 mg/day) and inhalation exposure (0.06 mg/day). Antacids and buffered aspirin can contribute on the order of thousands of mg/day to aluminium intake. Relative contributions to uptake are ranked similarly to these intake contributions. However, because inhaled aluminium is approximately seven times more bioavailable than aluminium in drinking water, the contribution of inhaled aluminium to uptake (1.7 × 10-5 mg/kg b.w./day) exceeds the corresponding contribution from drinking water (6.9 × 10-6 mg/kg b.w./day). Uptake of aluminium in food is approximately 1 × 10-4 mg/kg b.w./day. Aluminium uptakes from antacids and buffered aspirin amount to 3.1 × 10-1 and 4.3 × 10-2 mg/kg b.w./day, respectively.
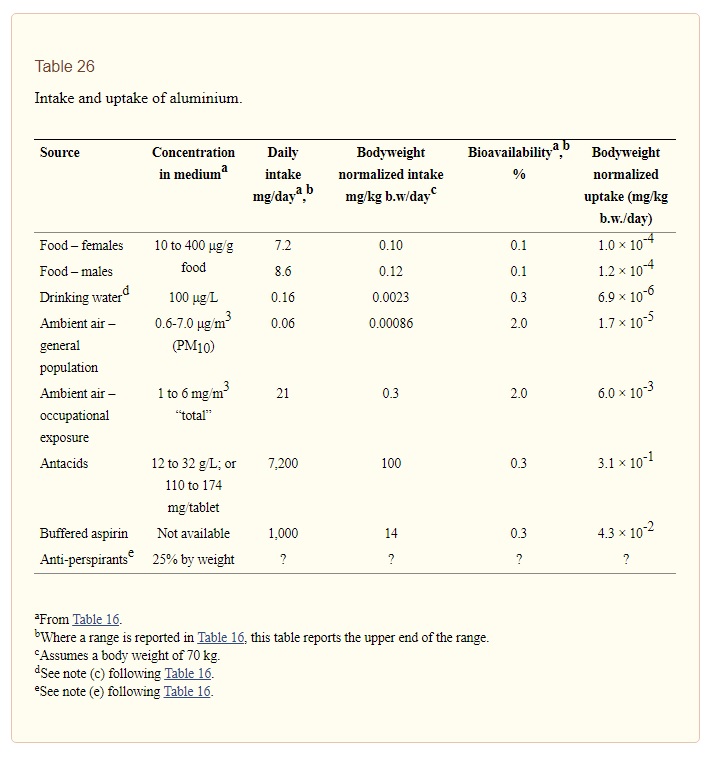
Relevant exposure levels of concern for the general population identified as part of dose response assessment included: irritation following inhalation (50 mg/m3), neurological effects due to drinking water exposure (100 μg aluminium/L water), reproductive toxicity due to oral intake (400 mg/kg-b.w./day), and irritation following injection (1 injection). We characterized risk (see Table 27) by calculating a margin of exposure, or MOE (the exposure level of concern divided by actual exposure), for each of these pathway-endpoint combinations. The MOE values were large for local irritation following inhalation (7000) and reproductive toxicity associated with oral intake (2900). For irritation following injection, the MOE is less than unity, although the severity of this endpoint is limited. For neurological effects associated with drinking water exposure, the MOE may be as small as unity. The evidence supporting this effect, however, comes from studies that have a number of methodological limitations, a finding that suggests the causal nature of the association is uncertain.