The Health Effects of Aluminum Exposure
Link: https://www.aerzteblatt.de/int/archive/article?id=193516&src=search
Dtsch Arztebl Int 2017; 114: 653-9. DOI: 10.3238/arztebl.2017.0653
Klotz, K; Weistenhöfer, W; Neff, F; Hartwig, A; van Thriel, C; Drexler, H
Background: Aluminum is regularly taken up with the daily diet. It is also used in antiperspirants, as an adjuvant for vaccination, and in desensitization procedures. In this review, we present the scientifically documented harmful effects of aluminum on health and the threshold values associated with them.
Methods: This review is based on publications retrieved by a selective search of the PubMed and SCOPUS databases on the topic of aluminum in connection with neurotoxicity, Alzheimer’s disease, and breast cancer, as well as on the authors’ personal experience in occupational and environmental medicine.
Results: The reference values for the internal aluminum load (<15 µg/L in urine, <5 µg/L in serum) are especially likely to be exceeded in persons with occupational exposure. The biological tolerance value for occupational exposure is 50 µg of aluminum per gram of creatinine in the urine. For aluminum welders and workers in the aluminum industry, declining performance in neuropsychological tests (attention, learning, memory) has been found only with aluminum concentrations exceeding 100 µg/g creatinine in the urine; manifest encephalopathy with dementia was not found. Elevated aluminum content has been found in the brains of persons with Alzheimer’s disease. It remains unclear whether this is a cause or an effect of the disease. There is conflicting evidence on carcinogenicity. The contention that the use of aluminum-containing antiperspirants promotes breast cancer is not supported by consistent scientific data.
Conclusion: The internal aluminum load is measured in terms of the concentration of aluminum in urine and blood. Keeping these concentrations below the tolerance values prevents the development of manifest and subclinical signs of aluminum toxicity. Large-scale epidemiologic studies of the relationship between aluminum-containing antiperspirants and the risk of breast cancer would be desirable.
![]()
![]()
Aluminum has long been established in medical applications as, e.g., an adjuvant in vaccines and an agent against pathological hyperhidrosis with a low side-effect profile (1, 2). In recent years, however, there has been more focus on the at times highly uncritical public debate about the neurotoxic effect of aluminum and its potential carcinogenic effect. Headlines such as “First evidence that aluminum in deodorants can actually trigger breast cancer” suggest to the reader that there is a proven link. Therefore, the question arises from a scientific perspective as to how high the risk of adverse health effects due to aluminum exposure actually is. There are numerous publications relating to this question (see the review by Willhite et al. [3]).
Our article examines the question of whether environmental and therapeutic aluminum exposure increases the risk of disease. To this end, Alzheimer’s disease and breast cancer are taken as critical endpoints. Aluminum’s neurotoxic effects in humans and its embryotoxic effects in animal models have been proven (4).
From a preventive medicine perspective, exposure to foreign substances should always be kept as low as reasonably achievable (principle of minimizing). Aluminum, however, is found in the blood and urine of all humans. Particularly in cases where a foreign substance exceeds its reference value (the value of the 95th percentile in the general population), one asks from a medical perspective whether, and from what level, does the substance pose a concrete health hazard.
Methods
A selective literature search on aluminum in association with neurotoxicity, Alzheimer’s disease, and breast cancer was carried out in the PubMed and SCOPUS databases; the authors’ experience in occupational and environmental medicine was also included in the analysis.
Environmental, occupational, and treatment-related exposure
Background exposure
Aluminum is the third most abundant element in the earth’s crust and occurs naturally in the environment, foodstuffs, and drinking water.
It is also used in:
- Processed foods
- Materials and articles such as:
– Aluminum-containing food packaging
– Aluminum foils
– Cooking utensils and baking trays - Cosmetic products (including antiperspirants, sun creams, toothpaste)
- Drugs (antacid agents).
Only around 0.1% of orally ingested aluminum is absorbed from the gastrointestinal tract and is made bioavailable (5).
The tolerable weekly intake (TWI) set by the European Food Safety Authority (EFSA) of 1 mg aluminum/kg body weight (BW) in a 60-kg adult is in some individuals already exhausted or slightly exceeded as a result of estimated daily alimentary aluminum exposure of 1.6–13 mg (0.2–1.5 mg/kg BW/week) (5) (Table 1). Relative exposure in children is higher at up to 2.3 mg/kg BW/week. TWI levels are designed to be precautionary and long-term values for the general population. Exceeding these values does not mean that there is an acute health hazard. Moreover, aluminum exposure depends greatly on the route of exposure. Exposure via the gastrointestinal tract and intact skin is extremely mild in humans. Therefore, the TWI value is suited to only a limited extent to reflecting the organism’s aluminum exposure. Internal exposure, which can be determined from aluminum levels in urine or blood, is a significantly better measure for assessing aluminum-related neurotoxicity.
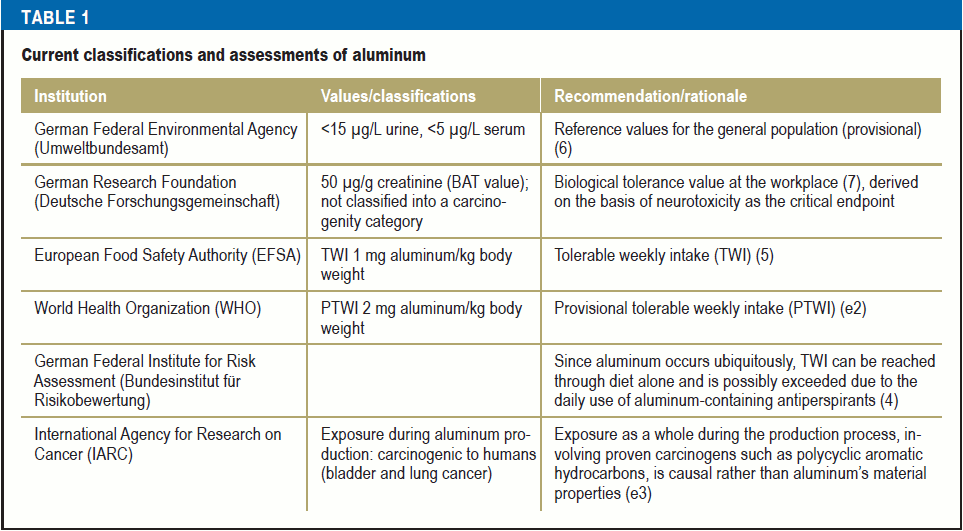
The literature reports widely differing values for the normal range of aluminum excretion, e.g., <7.5 µg/L plasma and <60 µg/24 h in urine (e1). Background exposure in the general population is put at <5 µg/L serum and <15 µg/L urine (provisional reference values set by the German Federal Environmental Agency [Umweltbundesamt]) (6). Table 1 provides an overview of the current classifications for aluminum. There are currently no studies that permit an evaluation of the different sources of internal exposure.
Occupational exposure
The internal exposure levels of those exposed in workplaces where aluminum welding is carried out, during electrolysis in aluminum production, or in the processing industries (e.g., foundries, powder production) can be significantly higher compared with individuals not exposed to aluminum at work, meaning that the reference values derived for the general population may be exceeded in these workers. Longitudinal studies on aluminum welders revealed that the aluminum content in welding fumes correlated with aluminum concentrations in blood and urine (8). The median plasma concentrations of approximately 10–14 µg/L are significantly below the plasma concentration of around 50 µg/L assumed to be the toxicity threshold in dialysis patients (8, e4).
The first subclinical changes detected using neuropsychological tests on a group basis was seen in aluminum welders in longitudinal studies over the 5-year study period at median aluminum concentrations post-shift of 120 µg/L (100 µg/g creatinine in urine) and 13 µg/L plasma (8, 9) compared with production workers not exposed to aluminum.
Workers in aluminum powder production in whom early-stage aluminosis was detected exhibited significantly higher aluminum concentrations at 340.5 µg/g creatinine and 33.5 µg/L plasma compared with controls (135.1 µg/g creatinine and 15.4 µg/L plasma) (10). Neurotoxicity was not investigated.
Aluminum in therapeutic applications
Antiperspirants: Aluminum compounds have been used commercially in antiperspirants since as early on as in 1903. Due to their antiperspirant effect, aluminum salts are used in dermatology at significantly higher concentrations (10–30% aluminum chlorohydrate) than in over-the-counter antiperspirants. The German Dermatological Society (Deutsche Dermatologische Gesellschaft) considers these to be a simple and suitable treatment option for hyperhidrosis with low side effects (2). Alternatives in the treatment of hyperhidrosis include tannin preparations with an astringent action, techniques such as tap water iontophoresis, chemical denervation with botulinum toxin A, systemic therapies with antihidrotic agents or psychotropic drugs, as well as surgical procedures (2).
Although aluminum is absorbed through the skin (11, 12), the penetration rate of aluminum chlorohydrate following the dermal application of antiperspirants is extremely low at around 0.01% (in two subjects [11]) and up to 0.06% in pre-damaged skin (in vitro [13]). To date, there are no epidemiological studies on internal exposure due to the use of antiperspirants following underarm shaving or the use of hair removal products.
Vaccination and hyposensitization: Aluminum salts are used as adjuvants in preparations for vaccines and hyposensitization. The adsorption of antigens on poorly soluble aluminum hydroxide augments the immunological effect (e5, e6). An aluminum dose of 0.1–0.8 mg is absorbed upon one-off application of a vaccine approved in Europe (14). Hyposensitization products approved for the German market contain 0.1–1.1 mg aluminum hydroxide per dose. Since these products are usually injected monthly over a 3-year period, aluminum exposure is significantly higher compared with a single vaccination.
Following injection, the aluminum salts become systemically available—the possible risks of this are currently the subject of critical discussion. In 2014, the Paul-Ehrlich Institute classified the “contribution of treatment with aluminum-containing therapeutic allergens to the lifelong accumulation of aluminum in the organism compared with aluminum exposure from other sources as low” and considers it acceptable in view of the therapeutic benefits (1). However, data on blood or urine levels in affected patients, which would enable an assessment of the risk of subclinical neurotoxic effects of aluminum, are lacking.
Health effects
The acute toxicity of aluminum is low (e7). No acute effects due to dietary exposure to aluminum have been observed in the general population (e7).
Table 2 shows examples for the long-known aluminum-related chronic diseases, aluminosis and dialysis encephalopathy syndrome, as well as for chronic disorders currently discussed in connection with aluminum exposure: Alzheimer’s disease and breast cancer.
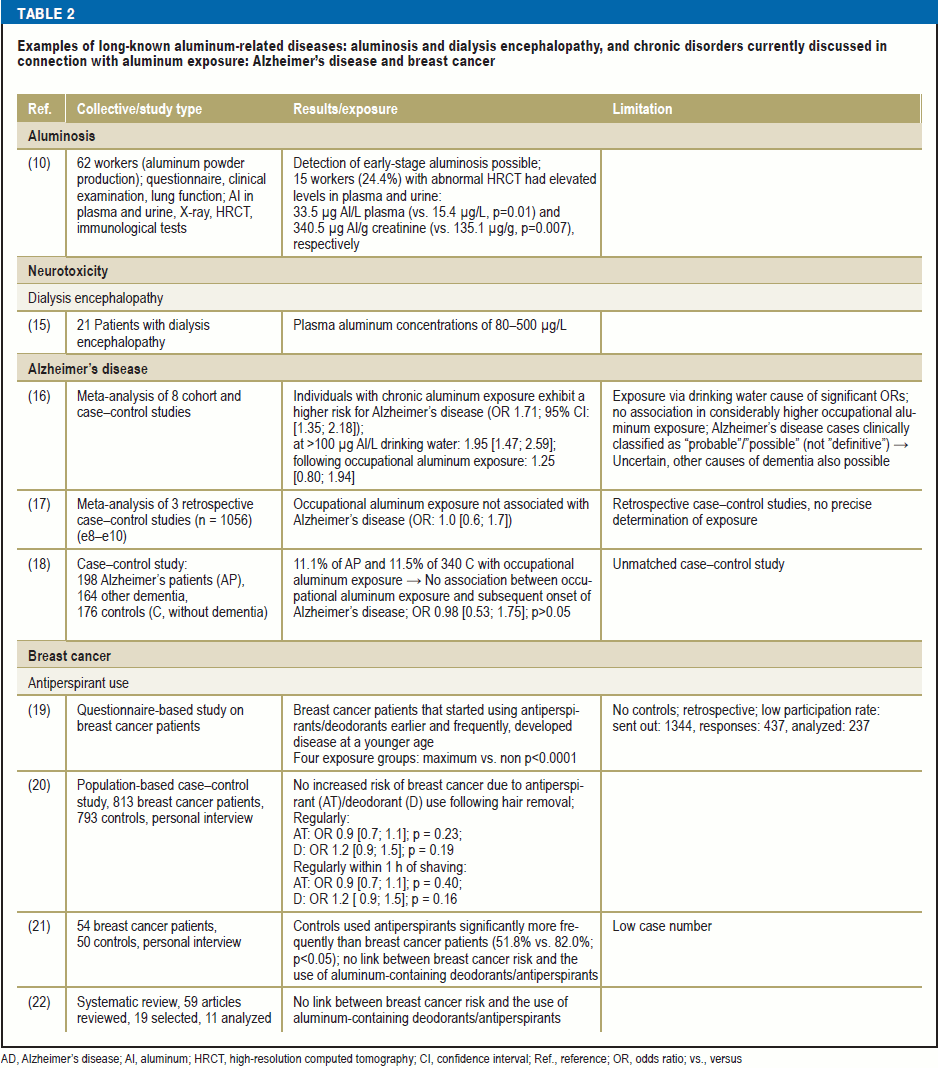
Neurotoxicity of aluminum
Aluminum (Al3+) exhibits a high affinity to proteins, which it is able to cross-link. In contrast to other ubiquitously occurring metals such as iron, manganese, and zinc, aluminum is not known to perform a physiological function in the human organism (23). Clinically relevant, neurotoxic effects have been described in dialysis patients. Aluminum salts, which were formerly added to the dialysate as a phosphate binder, were identified as the causal agents (15). Patients exhibited elevated aluminum concentrations in plasma and brain tissue (15, 24). Those affected showed disorientation, memory impairments, and, at advanced stages, dementia (15). The cause of these effects lies, firstly, in the slow—compared with other organs—removal of aluminum from the brain (e11) and, secondly, in the multitude of biological processes affected by aluminum in the brain (23).
In addition to inducing oxidative stress and binding to negatively charged membrane structures in neurons, aluminum is able to modify hippocampal calcium signal pathways that are crucial to neuronal plasticity and, hence, to memory (e12). Cholinergic neurons are particularly susceptible to aluminum neurotoxicity, which affect synthesis of the neurotransmitter acetylcholine (e13). Particularly the latter two neurobiological effects are also relevant in the presumed association between aluminum and Alzheimer’s disease (the Alzheimer’s hypothesis) (23). Aluminum-related neurotoxic effects could be partially reversed once aluminum contamination was no longer present in the dialysate (15).
Changes in neuropsychological tests (e.g., in relation to concentration, learning, and memory) were observed following occupational exposure of workers in whom concentrations of approximately 100 µg aluminum/g creatinine and approximately 13 µg/L plasma were measured; the neurotoxic effect of aluminum is considered causal here (8, 9, 25, 26) (Table 3). However, even following aluminum exposure above this threshold, no cases of manifest encephalopathy involving disorientation, impaired memory, and dementia have been reported (8–9, 10, 26, 27, e14). Only a single case report dating back to 1962 is available on one worker in whom rapidly progressive encephalopathy was observed and potentially linked to the patient’s concomitant aluminum fibrosis of the lung (28).
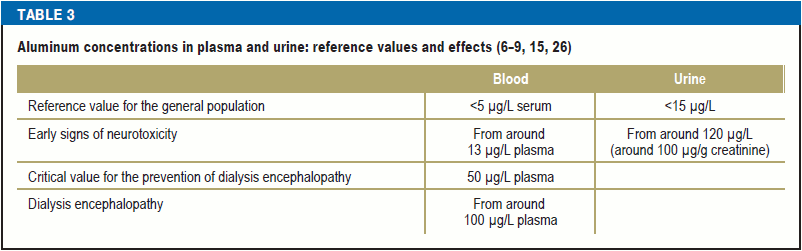
Is there a link between aluminum and Alzheimer’s disease?
In the course of the search for the causes of the frequently seen Alzheimer’s dementia, the described dementia syndrome following aluminum poisoning was also proposed as an explanation. Dialysis patients exhibited impaired speech, apraxia, and, in the further course, dementia syndrome as well as partly focal, partly generalized seizures (15). Specific EEG changes in the form of alternating spikes (2–3 c/s) and slow waves have proved to be characteristic and diagnostically significant (e15). Neuropathological investigations revealed minimal changes (mild hydrocephalus, only slight neuronal cell loss in the cortex, hippocampus, or Purkinje cells); mild vascular changes or aluminum detected in tissue have occasionally been reported (15), without evident changes typical of Alzheimer’s disease being identified (amyloid plaques and neurofibrillary tangles).
By contrast, in Alzheimer’s patiens the characteristic changes typical of aluminum encephalopathy were not observed. It was shown in several studies that an elevated aluminum content could be detected in the brains of Alzheimer’s patients, frequently in the endothelial cells of the walls of small and very small arteries, often associated with cerebral amyloid angiopathy (CAA) (e16), as well as in the central region of senile plaques (29).
The onset of Alzheimer’s pathology (both neurofibrillary tangles and amyloid plaques) was observed in animal models following intracranial/intraventricular administration of aluminum compounds (e17–e19). On the other hand, intraperitoneal or oral administration mostly produced no significant pathologies (e20, e21).
Wang et al. (16) found an increased risk for Alzheimer’s disease in their meta-analysis of individuals chronically exposed to aluminum in drinking water. In contrast, several studies found no association between aluminum exposure and Alzheimer’s disease after significantly higher occupational aluminum exposure (16–18) (Table 2).
From a critical perspective, the following can be concluded on aluminum exposure and Alzheimer’s disease:
- Aluminum can cause (in the case of extreme exposure) specific encephalopathy with a dementia syndrome.
- This aluminum encephalopathy is a distinct disease entity and is not the same as Alzheimer-type dementia.
- Elevated aluminum concentrations can be detected in the brains of Alzheimer’s patients. However, it is unclear whether aluminum is the cause of the change, or whether a secondary, independent change (apposition) takes place due to the Alzheimer’s pathology.
- Epidemiological studies provide only very uncertain indications of an association between aluminum exposure and Alzheimer’s disease.
Is there an association between aluminum and breast cancer?
For some time, there has been a discussion on whether the use of aluminum-containing antiperspirants can cause breast cancer (30). Although tumors are more frequently diagnosed in the upper outer quadrants of the breast, i.e., in close spatial proximity to where the substances are used, this is also an area with more glandular tissue (31–37). Nevertheless, an increase in this localization has been observed in recent decades (38). However, analysis of 746 consecutive breast tissue specimens showed that the percentage of diagnoses of normal, benign, or malignant tissue changes was comparable between quadrants (39).
Elevated levels of aluminum were also observed in the nipple aspirate fluid from female patients with breast cancer (e22), and likewise in an analysis of malignantly changed breast tissue (e23), whereby concentrations were higher in the outer compared with the inner quadrants (e24). However, aluminum does not appear to be the trigger of the tumors, but instead is stored to a greater degree in tumor tissue, much like other minerals. For example, feeding rats with a carcinogenic, non-aluminum-containing substance (2,7-dimethylbenz[a]anthracene) caused mammary gland tumors in which significantly elevated levels of aluminum were measured (40). Furthermore, besides aluminum, significantly elevated concentrations of other minerals (e.g., Cd and Ni, as well as Br, Ca, Cl, Co, Cs, Fe, K, Mn, Na, Rb, and Zn) were observed in human breast tumor tissue specimens (e25–e27).
In a more recent study, long-term exposure to aluminum chloride transformed breast epithelial cells in vitro in such a way (e.g., by increased DNA synthesis and DNA double-strand breaks) that the cells formed tumors and metastasized in an animal experiment (e28), which can be considered evidence of cell transformation.
A retrospective study showed an earlier age of disease onset in breast cancer patients that had used aluminum-containing antiperspirants combined with underarm shaving (19), whereas case–control studies (20, 21) failed to identify a link between the use of antiperspirants and the risk of breast cancer. Likewise, a systematic analysis of the published literature revealed no increased risk of breast cancer due to antiperspirant use (22).
In summary, there are currently no consistent data from epidemiological studies relating to an association between aluminum exposure and breast cancer risk; the majority of studies available to date found no association in this regard (Table 2). Collecting data on the use of aluminum-containing antiperspirants and breast cancer risk as part of a study with a longer observation period and high case numbers, like the German “National Cohort” (Nationale Kohorte), could yield more information. In addition, further mechanistic studies are needed.
Conclusion
The assessment of measured values in terms of their relevance to health is an important task in medicine. The neurotoxicity proven in humans and animals is the critical adverse effect of aluminum. This includes specific encephalopathy with a dementia syndrome, which, however, is not identical to the pathophysiology of Alzheimer-type dementia. A carcinogenic effect of aluminum has not been proven to date. It is possible to assess whether critical internal exposure levels are present from aluminum concentrations in blood and urine. Occupational health investigations are helpful here, since they describe experience gained in highly exposed groups. The available occupational health studies show, as a whole, that adverse neurotoxic changes are unlikely in the case of urinary excretion of <50 µg aluminum/g creatinine, even following long-term exposure.
Conflict of interest statement
The authors are active for the Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area of the German Research Foundation.
Manuscript received on 8 December 2016, revised version accepted on
21 January 2017
Translated from the original German by Christine Schaefer-Tsorpatzidis
Corresponding author
Hans Drexler,
Friedrich-Alexander-Universität Erlangen-Nürnberg
Institut und Poliklinik für Arbeits-, Sozial- und Umweltmedizin
Schillerstr. 25,
91054 Erlangen, Germany
hans.drexler@ipasum.uni-erlangen.de
