Suspected association of an allergic reaction with titanium dental implants: A clinical report
, , ,
https://doi.org/10.1016/S0022-3913(08)60233-4
Recent reports have questioned whether metal sensitivity may occur after exposure to titanium. This clinical report demonstrates the emergence of facial eczema in association with a titanium dental implant placed for a mandibular overdenture supported by 2 implants. Complete remission was achieved by the removal of the titanium material. This clinical report raises the possibility that in rare circumstances, for some patients, the use of titanium dental implants may induce an allergic reaction. (J Prosthet Dent 2008;100:344-347)
Recently, metal allergies from dental restorations, including allergies to nickel, iron, cobalt, and zinc, have been reported in association with oral lichen planus,1-3 hand eczema, or palmoplantar pustulosis.4-6 In contrast, titanium is considered to be a biocompatible material.7 However, in light of recent clinical reports regarding contact dermatitis or granulomatous reactions to titanium upon its use in pacemakers,8-11 hip prostheses,12-15
surgical clips,16 or osteosynthesis,17 there is debate with regard to titanium allergies. In addition, titanium has also been reported to possibly cause generalized health problems,18 and the potential for adverse human
tissue responses to titanium dioxide, which always covers the surface of titanium materials, has been reported.19 Nawaz and Wall recently reported on a patient demonstrating a drug rash with eosinophilia and systemic
symptoms (DRESS) syndrome, which reflects a serious hypersensitivity reaction to drugs, in association with titanium bioprosthetic implants.20 Sensitivity to titanium is characterized by the local presence of abundant
macrophages and T lymphocytes and the absence of B lymphocytes, indicating Type 4 hypersensitivity.21,22 Studies evaluating the oral tissue changes adjacent to titanium implants23 or titanium plates24,25 in patients reported no evidence of inflammatory response and no association between the identification of pigmented debris in the tissues and clinical symptoms.
However, the relationship between titanium dental implants and clinically relevant hypersensitivity has been recently suggested.26,27 These reports raise the question that metal sensitivity may arise after exposure to titanium for some patients in certain circumstances. This clinical report demonstrates the emergence of eczema localized to the perioperative facial area after receiving titanium dental implants, in which a complete emission was subsequently achieved by the removal of the titanium material.
CLINICAL REPORT
In May 1998, a 50-year-old Japanese woman was referred to the Ko Dental Clinic, Osaka, Japan, with a 2-year history of inflammatory skin lesions on her face. A physical examination revealed persistent skin rashes
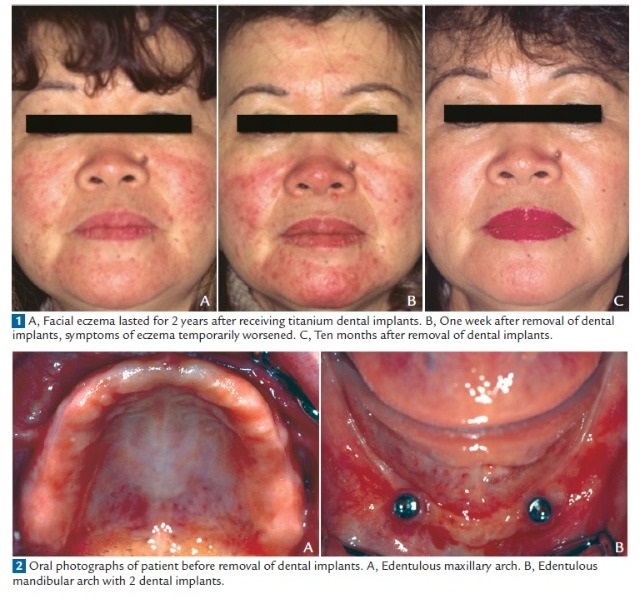
characterized by extremely red and itchy patches disseminated over her face; clinically, the signs were typical of eczema (Fig. 1). Laboratory data and complete blood counts were within normal limits. She had neither
systemic diseases nor previous contact or atopic eczema, nor hypersensitivity reactions to metals. She was edentulous and wore a complete maxillary denture and a mandibular overdenture supported by 2 implants (Fig. 2).
A detailed medical and dental history revealed that, 2 years earlier, the patient had received 2 dental implants in her mandible (Osseotite Implant System; Biomet 3i, Palm Beach Gardens, Fla). One week after implant
placement, rashes appeared on her mandibulofacial skin, and they gradually disseminated over her entire face. Although laboratory tests of urine and blood were normal and the patient exhibited no other symptoms, the eczema persisted.
Chronic low-level metal exposure may occasionally result in metal sensitization and undesirable side effects.28 Since the dental implants, which were purported by the manufacturer to be made of ASTM grade I high-purity titanium (99.64%), were the only extraneous substance chronically in contact with her body tissues, a possible relationship between the eczema and a titanium allergy induced by the dental implants was suspected. Patients that are affected by metal allergens are generally diagnosed using the epicutaneous (patch) test. However, such testing may induce falsepositive (irritative) reactions and may in itself sensitize or exacerbate
symptoms; therefore, the patient did not desire to undergo the patch test.
Alternatively, lymphocyte transformation tests (LTT), which have been recently validated for metal sensitivity testing, including testing for titanium hypersensitivity,18, 26, 29 were used. The LTT was performed as described in detail for detecting metal sensitivity.6,18 Briefly, 0.2 x106 peripheral blood mononuclear cells (PBMCs) in 200 μl of Roswell Park Memorial Institute (RPMI) medium 1640 (Nacalai Tesque, Kyoto, Japan) containing 20% autologous plasma were pipetted into the wells of a 96-well cell culture plate (Corning, Inc, Lowell, Mass). Antigen solutions of 4% TiCl3, 0.2% HAuCl4, 0.1% CH3COOAg, 0.2% PdCl2, 0.4% InCl3, 0.02% HgCl2, and 1% NiSO4 (Nacalai Tesque) were prepared in 6 dilution series (1/5n) each, and 20 μl of each antigen solution was added to the PBMCs in the culture well in triplicates. Following 72 hours of incubation at 37°C with 5% CO2, cells were pulsed for 18 hours with 10.6 KBq methyl-3H-thymidine (Moravek Biochemicals, Inc, Brea, Calif ) per well, and the radioactivity was measured in a liquid scintillation counter (Top-Count; PerkinElmer, Inc, Waltham, Mass). The counts per minute (cpm) were converted to a stimulation index (SI) representing the cpm in the test well divided by the average cpm in negative control wells. Conventionally,
a cutoff of SI ≥1.8 in at least 1 concentration of the 6 dilution series defined a positive reaction for the metals tested.6 LTT revealed a specific reaction to TiCl3, NiSO4, and HgCl2 with SImax of 2.39, 2.92, and 32.89,
respectively; all other tests were within the normal range (normal <1.8 for all materials tested). Based on the clinical history and the positive LTT index for titanium, the patient’s eczema was concluded to be induced by a titanium allergy.
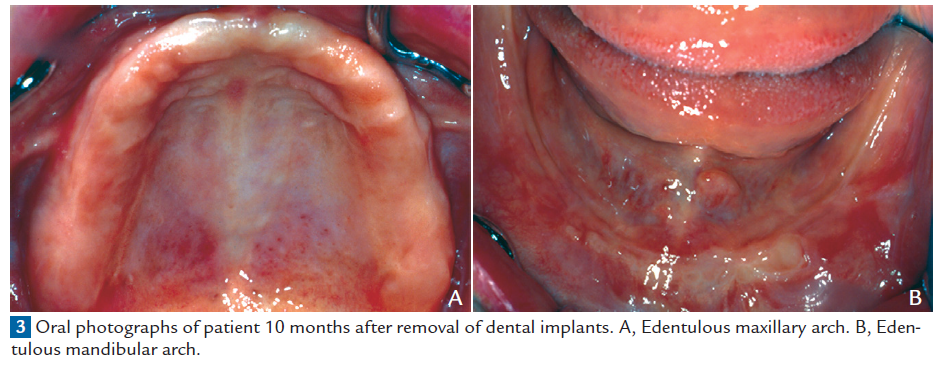
Therefore, all of the implants were surgically removed in July 1998 with the patient’s consent. One week later, the symptoms of eczema temporarily worsened (Fig. 1, B). However, the symptoms thereafter gradually improved, without requiring the prescription of any oral or topical medications, and complete resolution was achieved within 10 months (Fig. 1, C, and Fig. 3). The patient did not desire to undergo any further implant placement. Although the retention and stability of the mandibular denture were reduced without implants for support, the denture has since been managed by denture relining, and it has functioned acceptably to date. When the patient was last seen in April 2008, she was doing well and had no signs of recurrence.
DISCUSSION
Within the last 20 years, outcomes of treatment with dental implants have become increasingly predictable. Due to its recognized biocompatibility, titanium is not considered to provoke allergic reactions, although any foreign material has a potential to trigger an allergic response in certain circumstances. This clinical report demonstrates the emergence of eczema in association with titanium dental implants. A complete remission was achieved by the removal of the titanium material without oral or topical medications being prescribed. Due to both ethical and practical reasons, a reexposure of the patient to the suspected titanium allergen was not performed. However, it is noted that the symptoms of eczema temporarily worsened after the removal of the implants. This sudden turn for the worse in the patient’s condition suggests that the patient was rechallenged with titanium debris as an allergen during the titanium removal surgery. The patient did not have a history of dermatitis due to metal devices. At this time, there is no evidence that there is an increased risk of a reaction to an implanted device in patients who have skin patch or LTT sensitivity but no history of reaction to metallic materials. The importance of this line of investigation is increasing.30 Patients with a suspected titanium allergy from dental implants have been extremely rare, and this phenomenon has not yet been widely recognized in dental and medical fields. The history of titanium dental implant treatment is only a few decades old. Before such dental implant treatment became widespread, few people were exposed to titanium materials. This report suggests that dentists and physicians should be aware that the possibility of a titanium allergy from a dental
implant in some patients cannot be entirely eliminated.
SUMMARY
This clinical report presents a suspected association of an allergic reaction with titanium dental implants. It appears that in rare circumstances, for some patients, the titanium used in dental implants may induce an allergic
reaction. The rare occurance of such a response to titanium materials in clinical dentistry should, therefore, be further discussed and investigated.
